Corrective Action Forms
Tips and Tricks
As a quality professional we must know when to use corrective action forms. We use corrective action preventive action to support our business processes. We work within our company as the voice of the customer but we should not inhibit progress and process improvements. Therefore, we should not use CA forms for every known problem.
Its not practical to issue corrective actions for all non conformities. Management cannot support corrective action preventive action for all issues, for there are not enough resources to go around.
8D Manager Software with 8D, 9D, 5Y and 4M report generator. Your corrective action software for managing, measuring, and reporting issues.
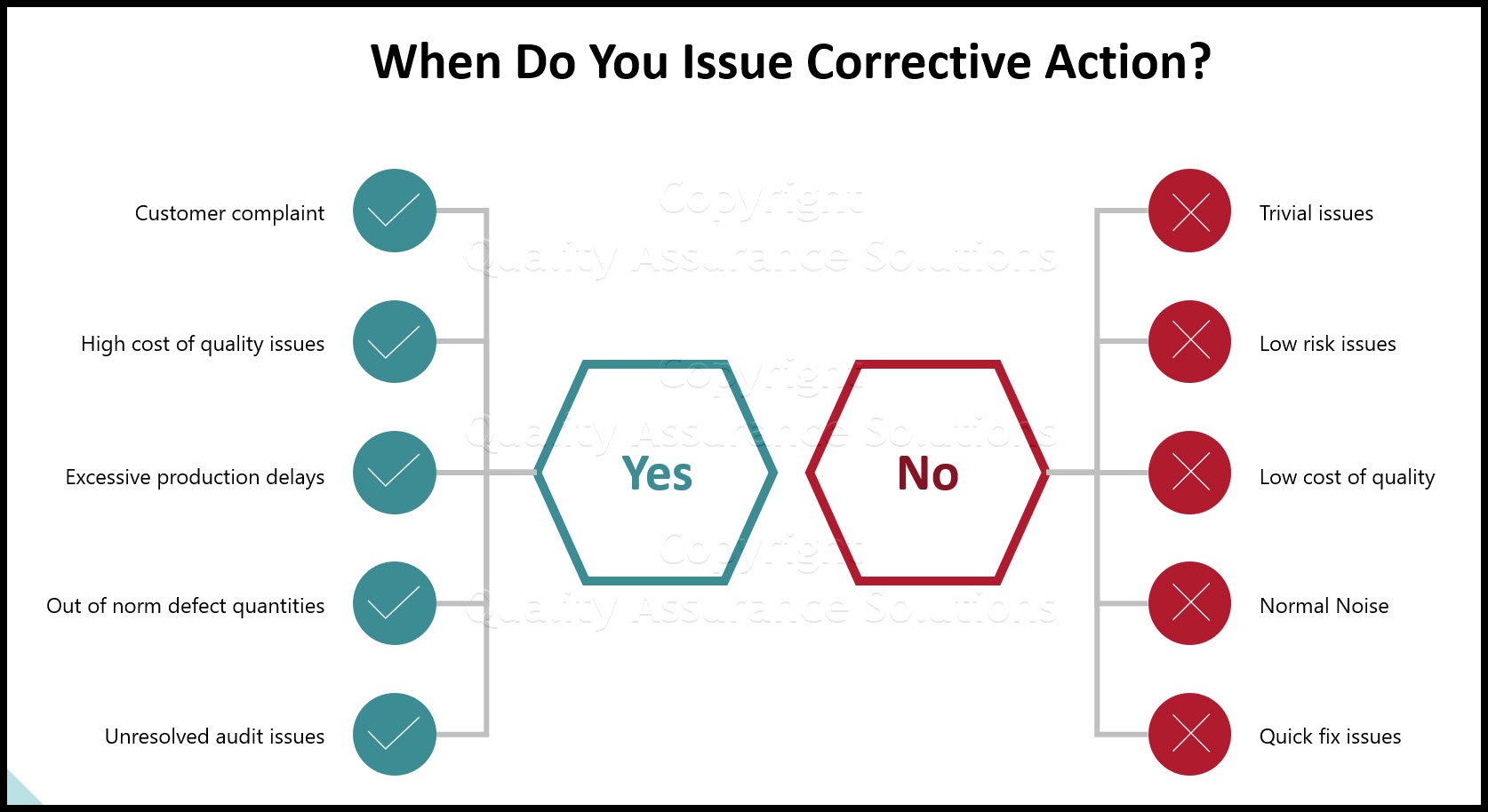
When do you issue corrective action?
How do you decide which issues need corrective action? I recommend issuing corrective action forms based on these issues
- Customer complaint
- High cost of quality issues
- Excessive production delays
- Out of norm defect quantities
- Safety related issues
- Unresolved audit issues
When do you not issue corrective action forms?
- Trivial issues
- Low risk issues
- Low cost of quality
- Normal Noise
- Quick fix issues
With regards to ISO 9001, corrective action does not need to be implemented for all known issues. ISO 9001 requires you to evaluate the need for action and implement corrective actions when you determine the necessary need. This gives you the choice on when to implement corrective action.
NCR Requirements and Corrective Action Forms
NCR Requirements and Corrective Action
Do you need to issue corrective action forms for every non conformance report (NCR) or every product defect? This depends on your industry. In general here are some guidelines.
Per ISO 9001 you need to document the non conformance. You need to evaluate the need for the corrective action of that non conformance. You can do this on the non conformance form. Or, you can document a set of rules when you create a corrective action. You then judge the non conformity based on those rules.
For example your corrective action rules could include:
- Failures on an SPC chart
- Failures beyond a certain company costs
- Failures beyond a certain number of units
- Failures that cause a safety issue
- First time failure only
- Failures that effect reliability
- Field Failures
8D Manager Software with 8D, 9D, 5Y and 4M report generator. Your corrective action software for managing, measuring, and reporting issues.
Implementing an Effective Corrective Action System
Do not implement corrective action forms for every known issue or product defect. Excessive corrective actions slow down the implementation of true corrective action. Instead of chasing paperwork, focus on the root cause of the important issues. The act of the corrective action needs to benefit your company.
When designating someone as the responsible person for the corrective action, they must have the authority of implementing change. They must be able to work with the parties that created the issue. The person with authority means that he / she can issue warnings / reprimands to those parties not following the corrective action plan. This “power” is needed to assure closing the corrective action.
In addition to authority, the leader of the corrective action effort needs to have top management support. Top management needs to review, promote, support and encourage the corrective action efforts. Top management reviews the corrective action to ensure there is no conflicting plans. The executive team promotes corrective actions which lets the corrective action team and other company employees know that the CA is critical to the company. Top management supports the efforts by eliminating roadblocks to the actions. Top management encourages the corrective action process to assure the members stay focus as they understand the elimination of the issue is important to the business.
Select the correct people for the corrective action. The team must be knowledgeable of the process details that causes the problems. Someone within the team must have the authority to make and enforce changes.
8D Manager Software with 8D, 9D, 5Y and 4M report generator. Your corrective action software for managing, measuring, and reporting issues.
Measuring Corrective Action
Some quality system standards require you measure corrective action. Below is a list of possible measurements with regards to monitoring corrective action..
- Number of open issues
- Number of closed issues
- Number of open / total number for a given period of time
- Number of overdue CA
- First time failure only
- Average amount of closure time
- Number of corrective action forms per type of issue
- Number per root cause issue
- Number of CA per Quality System element
- Number per given process
- Age of the corrective action
- Number of CA that did not resolve the problem
- Number of NCR for the same issue after completion of the CA
- Amount of money spent on a CA
- Number of canceled corrective actions
- Number of NCR / CA per a time period
- QAS Home
- 8D Reports
- Corrective Action Forms Tips and Tricks
|
Quality Assurance Solutions Robert Broughton (805) 419-3344 USA |
 |
|
Software, Videos, Manuals, On-Line Certifications | ||
|
450+ Editable Slides with support links | ||
|
Corrective Action Software | ||
|
Plan and Track Training | ||
|
AQL Inspection Software |
|
Learn and Train TRIZ | ||
|
Editable Template | ||
|
Templates, Guides, QA Manual, Audit Checklists | ||
|
EMS Manual, Procedures, Forms, Examples, Audits, Videos | ||
|
On-Line Accredited Certifications Six Sigma, Risk Management, SCRUM | ||
|
Software, Videos, Manuals, On-Line Certifications |