QA Project Plan
What is The Difference Between a Project Quality Management Plan and a QA Project Plan?
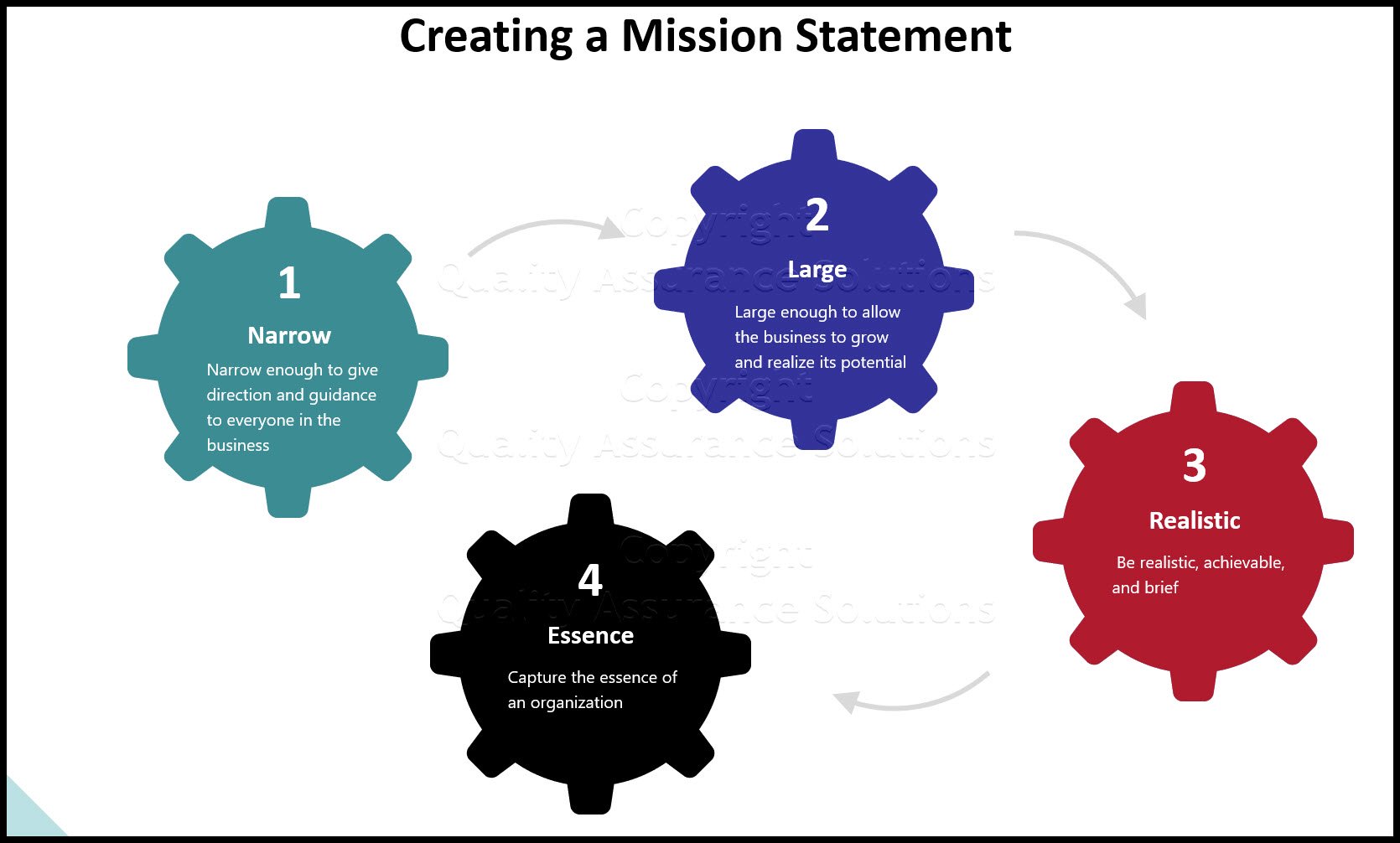
A QA Project Plan (QAPP) describes the necessary QA procedures, quality control (QC) activities, and other technical activities that will be implemented for a specific project or program.
A Project Quality Management Plan describes an organization's quality system,
i.e., its systematic approach to quality
assurance. The Quality Management Plan is also know as the Quality Management System, (QMS). ISO 9001 provides guidelines for one type of QMS.
What is a QA Project Plan?
A QAPP describes the activities of the data operations project involved with the acquisition of QA information whether generated from direct measurements activities, collected from other sources, or compiled from computerized databases and information systems.
What is The Purpose of The QAPP?
The QAPP documents the results of a project's technical planning process, providing in one place a clear, concise, and complete plan for the QA data operation and its quality objectives and identifying key project personnel.
Your ISO 9001:2015 Kit includes Templates, QA Manual, Implementation Guide and a Gap Assessment Internal Audit Tool for ISO 9001:2015
May I Combine a Project Quality Management Plan and a QA Project Plan into one Document?
Yes. With permission of the QA Manager of the organization sponsoring the work, these two documents may be combined into a single document for small programs, grants, and contracts. The combined document should address satisfactorily all the elements of both documents.
What Are The Benefits of a QA Project Plan?
The QAPP communicate, to all parties, the specifications for implementation of the project design and to ensure that the quality objectives are achieved for the project.
It does not guarantee success every time, but the prospects are much higher with a QA Project Plan than without one. Up-front planning eliminates approaches that do not work well (or not at all), which potentially reduces the cost of lost time and rework.
Implementation as prescribed, with appropriate QC practices employed, increases efficiency and provides for early detection of problems, either in the field or in the laboratory. This again saves time and money from the rework and enables the ability to make decisions more expeditiously. For example, following calibration procedures help assures the credibility and usability of data generated by laboratory instruments.
When Should a QAPP Plan Be Prepared?
Prepare a QAPP is prepared either part of or after the project planning process. But in all cases, the QAPP should be completed and approved before the starting the project.
PDCA Complete is an organizational task management system with built-in continuous improvement tools. Includes projects, meetings, audits and more.
Built by Quality Assurance Solutions.
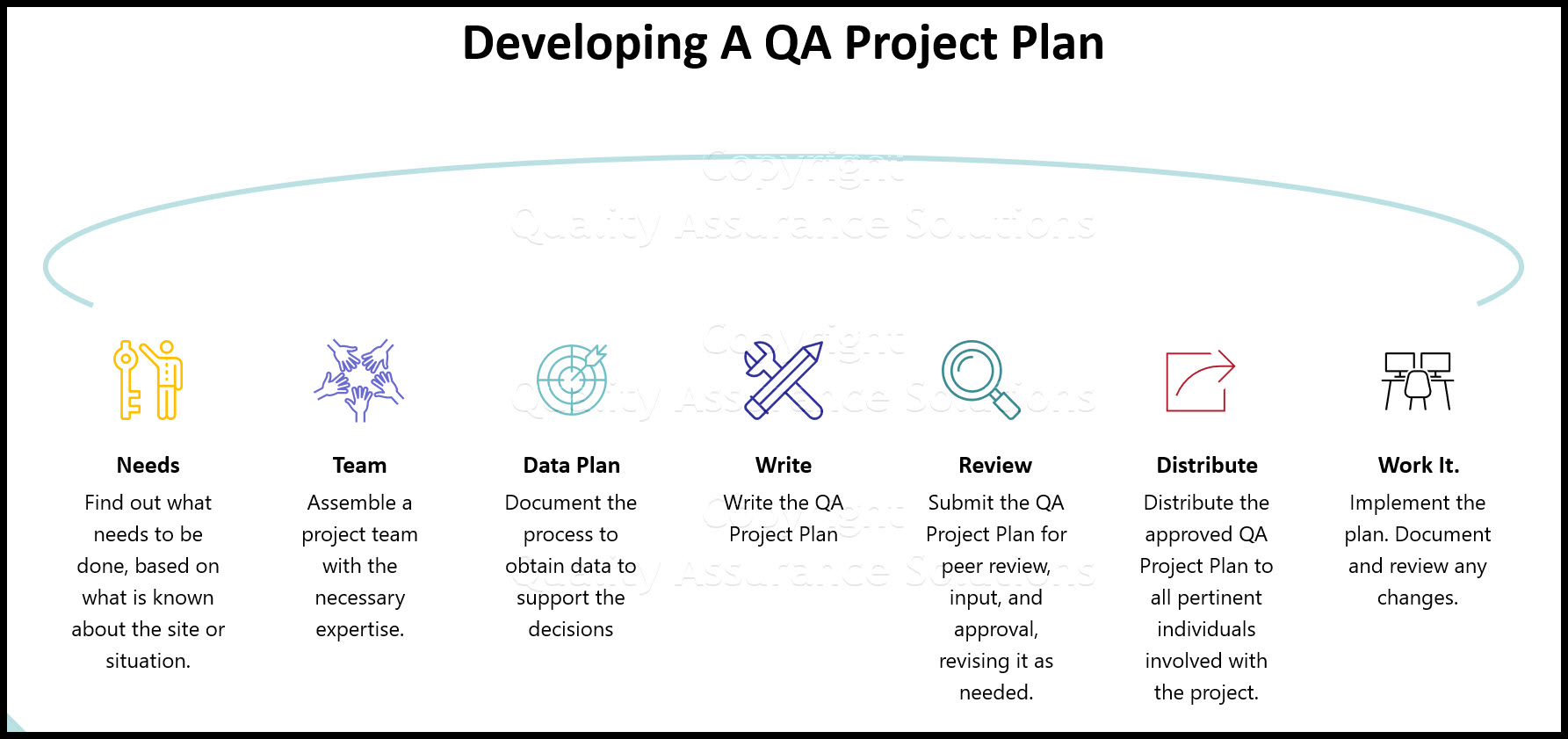
How Do I develop a QA Project Plan?
The following is a brief summary of the process:
- Find out what needs to be done, based on what is known about the site or situation.
- Assemble a project team with the necessary expertise.
- Plan what can be done, or what will be done to obtain data of known quality that are good enough to support the decisions to be made or the study questions to be answered.
- Write the QA Project Plan.
- Submit the QA Project Plan for peer review, input, and approval, revising it as needed.
- Distribute the approved QA Project Plan to all pertinent individuals involved with the project.
- Begin work while implementing the plan, but remember to: document any changes in the QA Project Plan, get re-approval before initiating the change, and then distribute the updated version.
Should a QAPP be approved before work begins?
Yes. Complete all work involving the collection or use of data with an approved QAPP. A QA Project Plan should generally be approved before any data collection operation starts.
Advance approval ensures that the team completed all the planning steps, including connecting actions with needs. Clear documentation increases the likelihood that the project achieves its intended results. If the plan is not approved before work begins, a stop-work order may be issued.
When Should I Revise my QAPP?
When changes affect the scope, implementation, or assessment of the outcome, revise the plan to keep project information current. The Project Manager, with the assistance of the QA Manager, determines the impact of any changes on the technical and quality objectives of the project.
For long-term projects, such as multi-year monitoring programs, review the QA Project Plan annually by the Project Manager to determine the need for revision.
PDCA Complete is an organizational task management system with built-in continuous improvement tools. Includes projects, meetings, audits and more.
Built by Quality Assurance Solutions.
When Should I Submit a Revised QAPP For Review?
When a substantive change warrants, the originator of the QAPP revises the plan to document the change, and then submits the revised plan to the approving authority. Implement the change only after approval of the revision. Send the revised plan to all the individuals cited in the distribution list.
How Long Do We Keep the QAPP After The Project Ends?
Document retention should comply with the approving organization's specifications first, and the specifications of the organization performing the work second.
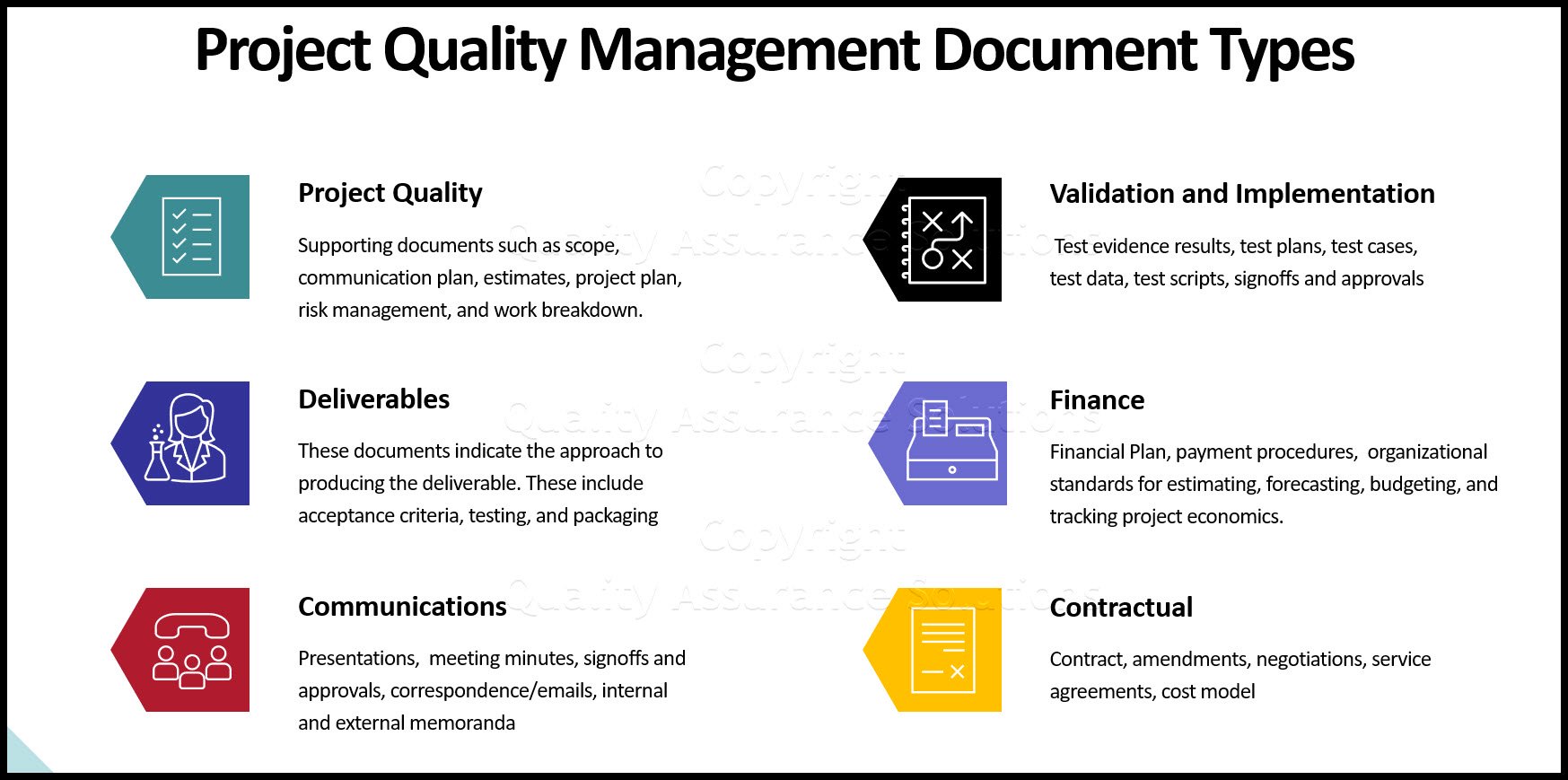
What is Generally Contained in a QAPP?
Divide the QAPP into four basic element groups:
- Project Management Data Generation and Acquisition;
- Assessment
- Oversight
- Data Validation Usability
A QAPP that addresses the basic elements defines and describes the following:
- Who uses the data;
- What are the project's
goals/objectives/questions or issue
- What decision(s) will be made from the information obtained;
- How, when, and where project information will be acquired or generated;
- What possible problems may arise and what actions can be taken to mitigate their impact on the project;
- Specify the type, quantity, and quality of
data;
- How "good" those data have to be to support the decision to be made;
- How the data will be analyzed, assessed, and reported.
What if some of the elements do not apply? Plans vary in their level of complexity, based both on the nature of the work being performed (such as the collection of new data or the use of previously collected information), available resources, and the intended use of the data.
PDCA Complete is an organizational task management system with built-in continuous improvement tools. Includes projects, meetings, audits and more.
Built by Quality Assurance Solutions.
Can Additional Information be Specified Beyond The Standard Elements?
The organization sponsoring or overseeing the work may specify additional information to clarify project-specific information.
If This Information is Documented in Other Places, Do I Rewrite That Information Into This QA Project Plan?
Referring to existing documents can reduce the plan preparation and review time and length. Any documents prepared before the QAPP, such as standard operating procedures (SOPs), sampling and analysis plans (SAPs), work plans, assessments, literature files, and data sets from other projects, may be appended. Alternatively, they may be incorporated by reference, if those sources are readily available to both reviewers and project personnel who implement the QAPP.
How Long is a QAPP?
A Plan should have enough information to describe project objectives and details. The number of pages needed to address this information varies with the complexity of the project and intended use of the information.
A plan for some QA data operations may involve a qualitative discussion of the experimental process and its objectives, while a plan that describes a complex project may involve extensive documentation to adequately describe activities.
May I Use The Same Plan For Standard Activities?
Multi-year projects, and projects conducted at multiple sites, containing the same project objectives and sampling and analytical processes, may be described in a generic QAPP. You may describe site specific activities in supplements, for example, separate field sampling plans. Review generic plans annually to determine if any changes are necessary.
What is The Role of Systematic Planning in Developing The QAPP?
Systematic planning
is a process in which you identify the problem to be investigated or the
decision to be made, and
then define the project's objectives, the type, quantity and quality of
information needed, the technical
and quality control activities, and the level of oversight to ensure
satisfaction of project criteria.
PDCA Complete is an organizational task management system with built-in continuous improvement tools. Includes projects, meetings, audits and more.
Built by Quality Assurance Solutions.
Who is Included in Developing The QAPP?
Project planning necessitates the coordinated efforts of many individuals, such as those who generates information and those who uses the information or make decisions based on that information. These individuals include:
decision makers,
project managers,
regulators,
stakeholders,
modelers,
risk assessors,
and technical staff (for example, hydrologists, chemists, data validators, samplers, and statisticians).
In addition, include peer reviewers and individuals with varied expertise to ensure it sufficiently addresses technical areas, thus helping to minimize problems during implementation.
Who Ensures That The Plan is Written?
Those who are both involved in planning the project and experienced in the operations, prepare and/or assist in the preparation of the QA Project Plan. For internal projects, the Project Manager or Principal Investigator is generally responsible for overseeing plan preparation. For externally funded projects, the recipient of the funds is usually responsible for project plan development.
Who Reviews The Plan?
This varies with each organization. Reviewers with expertise in the project specific areas, such as
- program managers (decision makers),
- QA staff independent of project management, and
- project field and laboratory technical staff, should review the plan.
What is Included in a QAPP Review?
Reviewers should:
- Ensure that the information is accurate and complete;
- Ensure that all appropriate elements are addressed;
- Ensure that the plan identifies the project's technical and quality objectives, and that the intended measurement and data acquisition methods will satisfy these objectives;
- Confirm that the planned assessment procedures will be adequate to evaluate the project
- Confirm that there is a process to identify any limitations on the use of the data.
These reviewers may also use tools, such as a checklist, in their review.
PDCA Complete is an organizational task management system with built-in continuous improvement tools. Includes projects, meetings, audits and more.
Built by Quality Assurance Solutions.
Who Approves The QAPP?
The approving authority will vary with the individual organization. The organization's Project Quality Management Plan establishes how, when, and by whom development, review, approval, and effective oversight of QA Project Plans should occur. This includes processes for extramural organizations that prepare QA Project Plans.
The Project Manager or Project Officer, and the QA Manager usually approves the QA Project Plan. For extramural projects, the responsible organization's Project Manager, or Principal Investigator, and QA Manager may review and approve the QA Project Plan, and then submit it for customer approval. It is also beneficial if other key staff, such as the laboratory directors and prime contractors and subcontractors, sign the plan to indicate their review and approval
What Types of Approvals Exist?
In situations where only non-critical deficiencies in a QAPP have not been resolved (such as a final organizational chart or a data analysis procedure that will not be followed for weeks), conditional approval may be given to allow the project to start while these deficiencies are being resolved. The plan is then resubmitted for approval when the information is finalized. The concept of conditional approval, however, varies with individual organizations; some organizations may not permit conditional approval of a QA Project Plan.
Who Gets a Copy of The QAPP?
All personnel involved in the project should retain or have access to the current version of the QAPP. This may include the Project Manager, laboratory manager, field team leader, modeler, QA Manager, data reviewers, and any essential contractor and subcontractor personnel involved with the project.
Who is Responsible For Implementing QAPP?
The organization performing the work is responsible for ensuring that the QAPP is implemented as written and approved, whether this work is conducted by contract personnel or in-house personnel. Ultimately the Project Manager is responsible for project activities. A clearly written QA Project Plan will help the Project Manager implement the plan, because all project personnel will understand the specifications before the start of data generation activities.
- QAS Home
- QMS Review
- QA Project Plan
|
Quality Assurance Solutions Robert Broughton (805) 419-3344 USA |
 |
|
Software, Videos, Manuals, On-Line Certifications | ||
|
An Organizational Task Management System. Projects, Meetings, Audits & more | ||
|
Corrective Action Software | ||
|
Plan and Track Training | ||
|
AQL Inspection Software |
|
450+ Editable Slides with support links | ||
|
Learn and Train TRIZ | ||
|
Editable Template | ||
|
Templates, Guides, QA Manual, Audit Checklists | ||
|
EMS Manual, Procedures, Forms, Examples, Audits, Videos | ||
|
On-Line Accredited Certifications Six Sigma, Risk Management, SCRUM | ||
|
Software, Videos, Manuals, On-Line Certifications |