Weight Scale Calibration Procedure Example
Below is an example of a weight scale calibration procedure for a Sartorius Scale. It is an example only. Use the scale's manual to determine the accuracy of the scale. You select which calibrated weights to use. These weights must be traceable to NIST. It is important to your Quality Assurance system that you have a calibration procedure in place for each measuring instrument.
Includes an easy to edit Calibration Manual, recommended calibration system, reports and templates.
Weight Scale Calibration Procedure
A) Scope: This procedure describes our company’s method for calibrating a Sartorius GP5202 Precision Balance.
B) Transfer Standard: Weight set ### consisting of 1 gram, 1000 gram, and 5000 gram weights. These are calibrated to NIST with an accuracy of .2%.
C) Scale Accuracy: The scale is accurate to 2% for each weight.
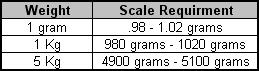
D) Scale requirements for each weight:
E) Weight Scale Calibration Process:
1.0 Check the scale in this controlled environment 23°C ± 2°C and relative humidity of 40% ±10%.
2.0 Tare the scale so display reads all 0s.
3.0 On the calibration record document the requirement for a 1 gram scale. See above.
4.0 Place the 1 gram weight on Scale and record the scale's reading on the calibration record next to the requirement. See below example:

Includes an easy to edit Calibration Manual, recommended calibration system, reports and templates.
5.0 Repeat steps 1.0 through 4.0 for the 1000 gram and 5000 gram weights.
7.0 If any of the weight readings are out of the scale requirements, immediately take the calibration record to the Quality Assurance Manager for their review. QA Manager documents the review findings on the calibration record.
8.0 Document these items on the calibration record:
- Calibration record number
- Scale Number
- Name of Scale
- MFG Name of the scale
- Scale Location
- Scale Calibration procedure # (this procedure)
- Calibration interval
- Name of Weights
- Weight Identification number
- Next calibration due date
- Temperature
- Humidity
- Accept / Reject decision checkbox
- Employee’s name or signature
9.0 Place a calibration sticker on the scale. Reference the calibration record number from the calibration record.
Includes an easy to edit Calibration Manual, recommended calibration system, reports and templates.
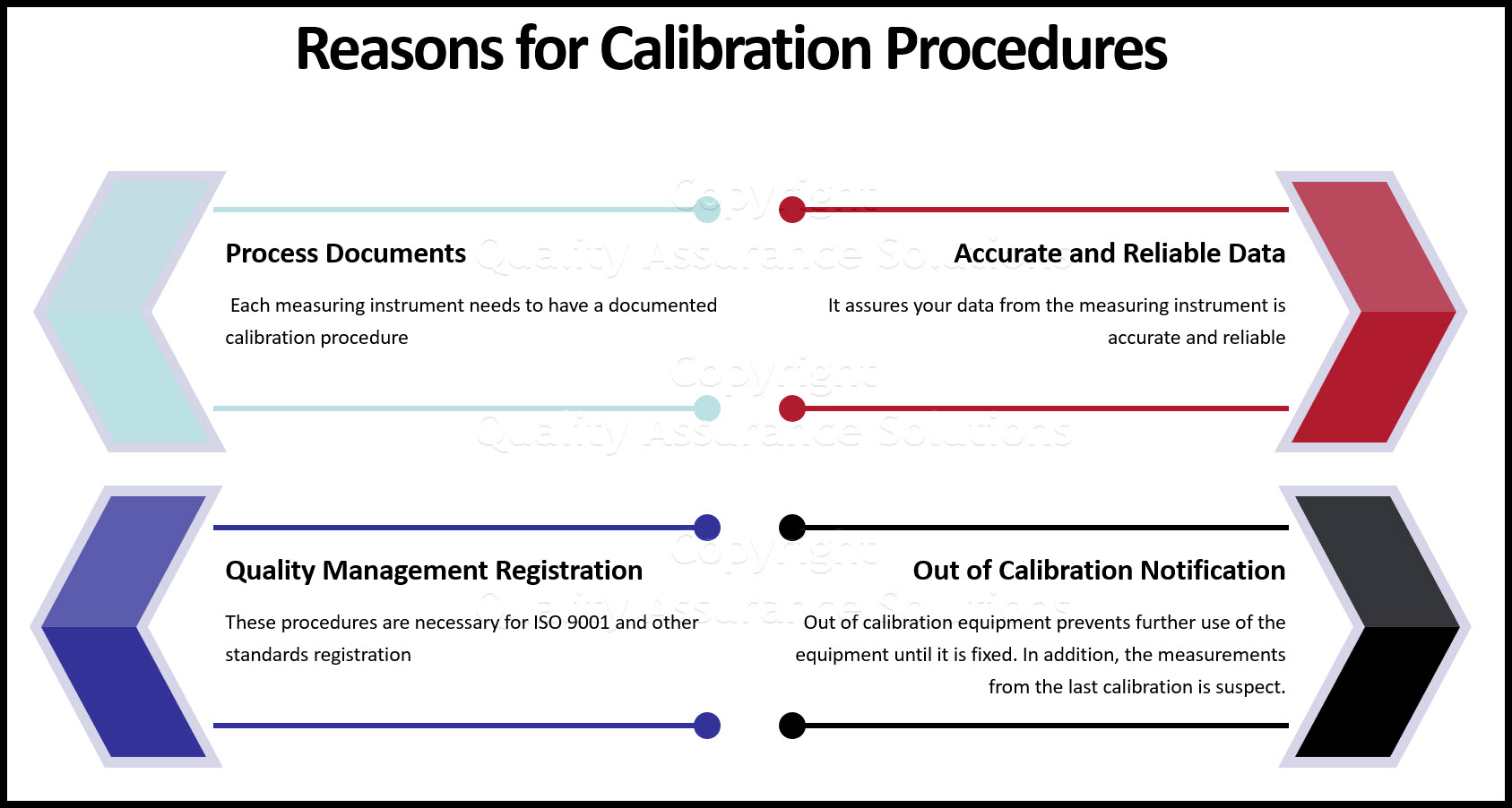
Procedures like these within your calibration department is important for the following reasons:
A) Each measuring instrument needs to have a documented calibration procedure
B) These procedures are necessary for ISO 9001 registration
C) It assures your data from the measuring instrument is accurate and reliable
D) If the equipment was out of calibration it will prevent further use of the equipment until it is fixed. In addition the measurements conducted by the instrument from the last calibration is suspect. You can identify these measurements, isolate suspected material and remeasure with an accurate calibrated equipment. You could issue corrective action for this issue.
- QAS Home
- Calibration
- Weight Scale Calibration
|
Quality Assurance Solutions Robert Broughton (805) 419-3344 USA |
 |
|
Software, Videos, Manuals, On-Line Certifications | ||
|
An Organizational Task Management System. Projects, Meetings, Audits & more | ||
|
Corrective Action Software | ||
|
Plan and Track Training | ||
|
AQL Inspection Software |
|
450+ Editable Slides with support links | ||
|
Learn and Train TRIZ | ||
|
Editable Template | ||
|
Templates, Guides, QA Manual, Audit Checklists | ||
|
EMS Manual, Procedures, Forms, Examples, Audits, Videos | ||
|
On-Line Accredited Certifications Six Sigma, Risk Management, SCRUM | ||
|
Software, Videos, Manuals, On-Line Certifications |