ISO 9001 2015 procedures
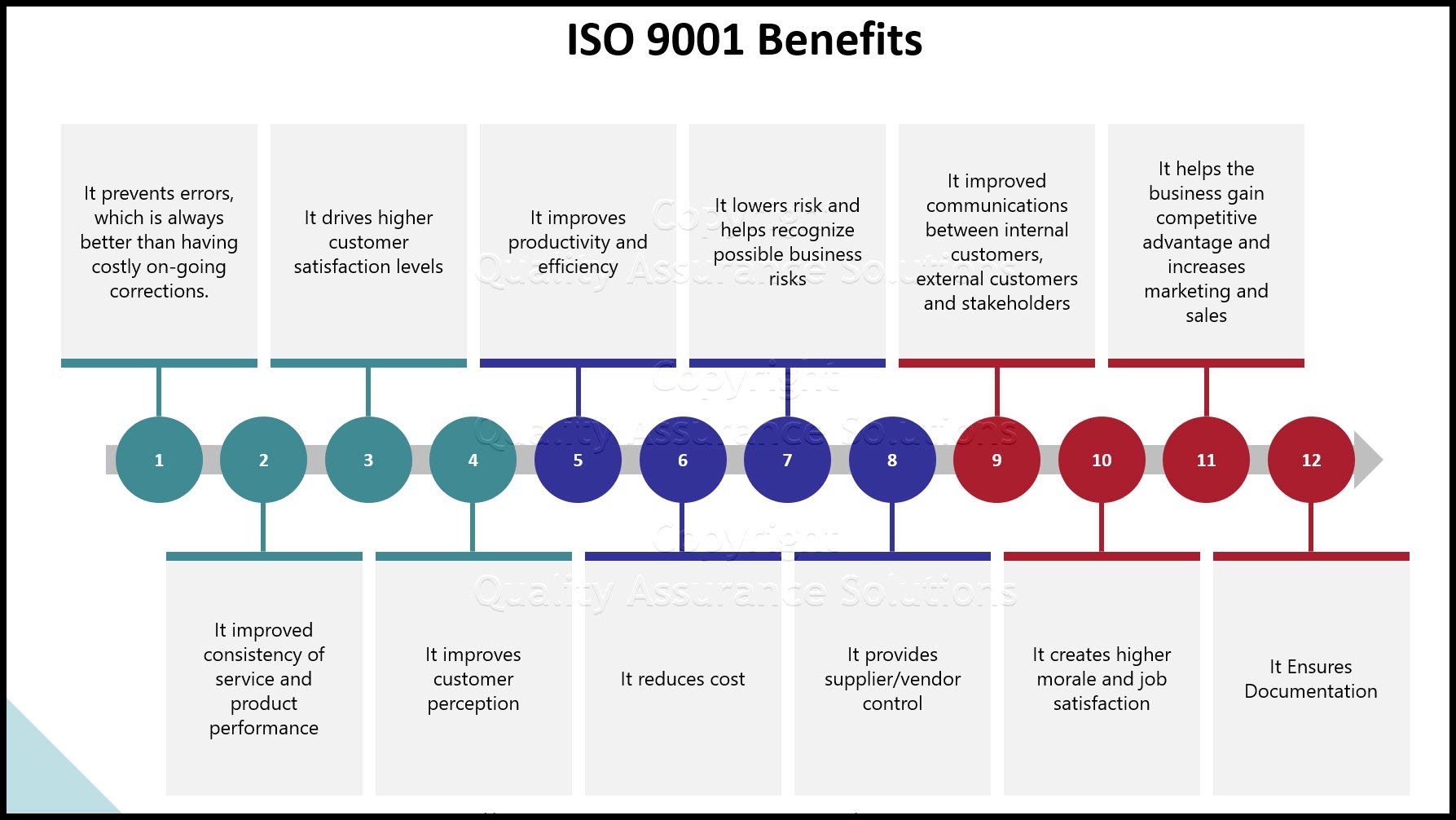
ISO 9001 2015 procedures require these documents to be in place. They are mandatory.
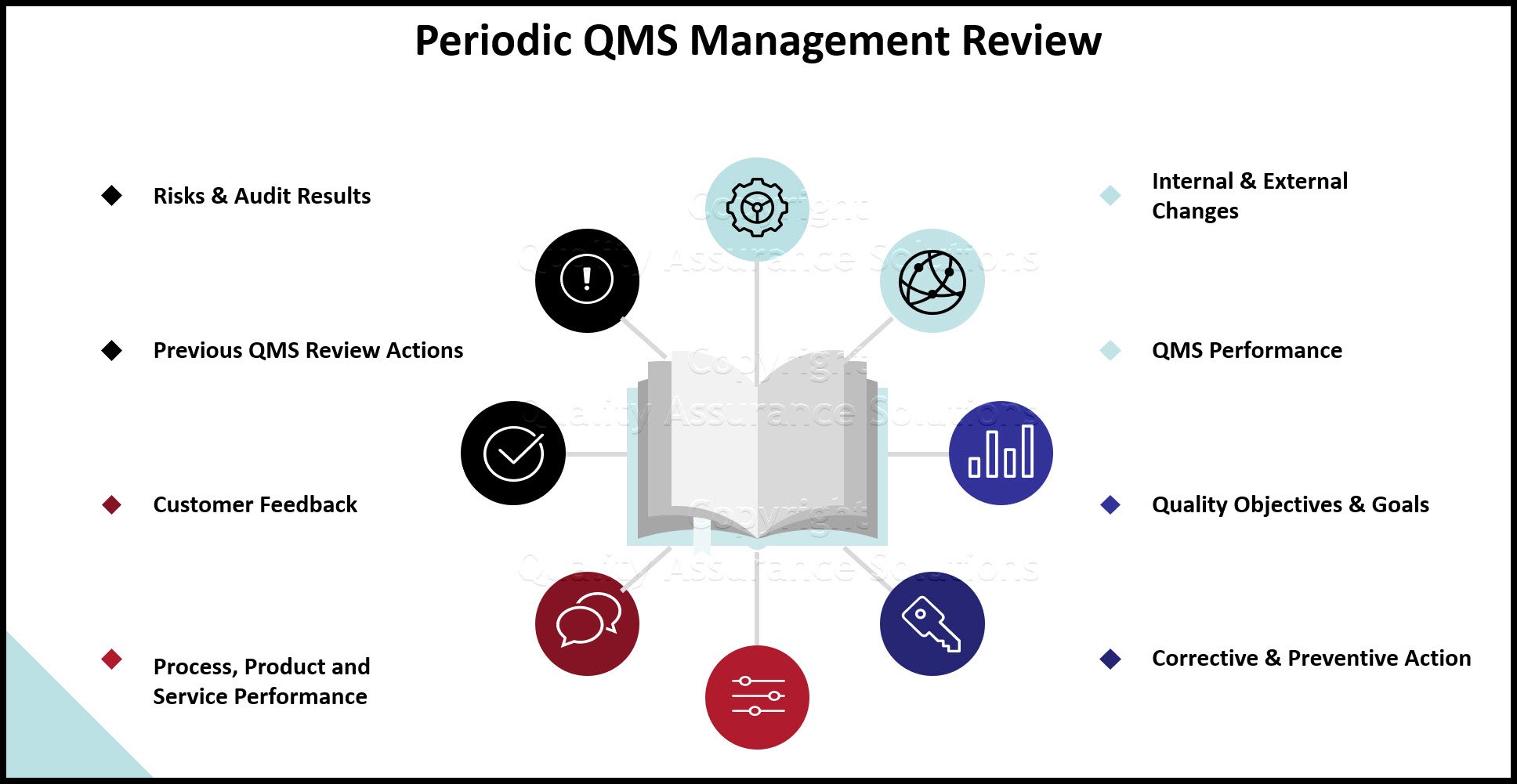
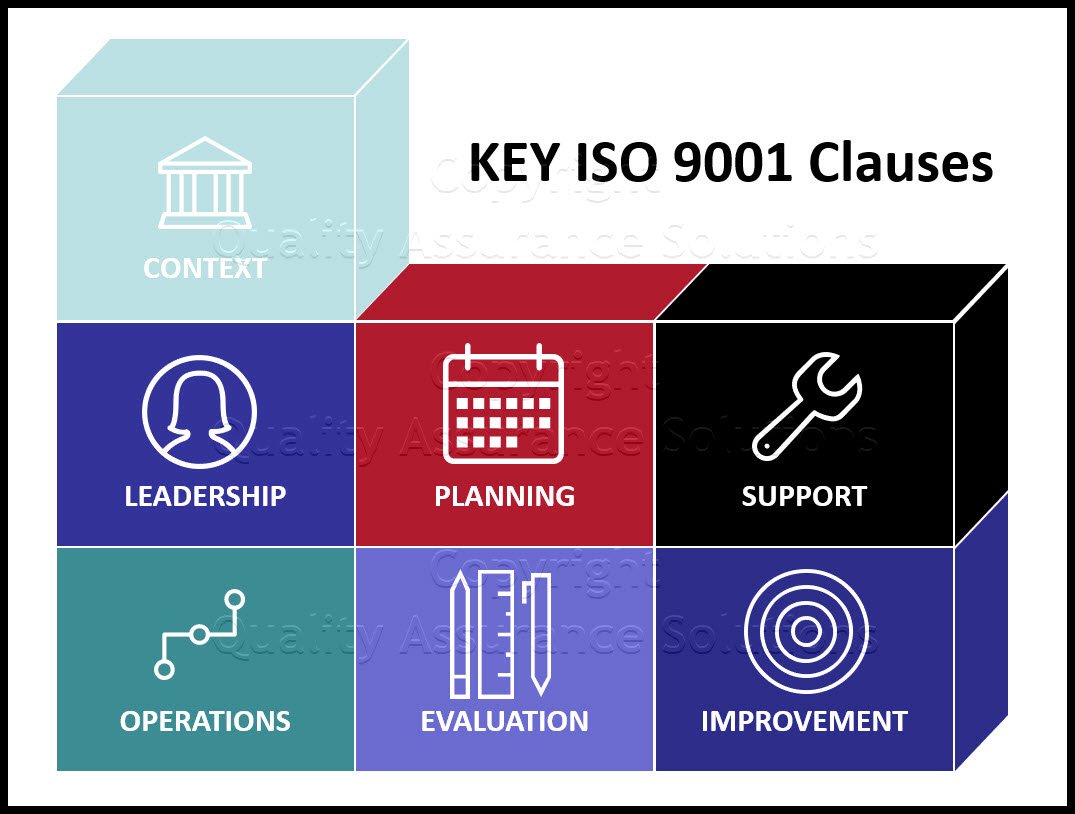
Documented information needed to be maintained by the organization for the purposes of establishing a QMS (high level documents) including:
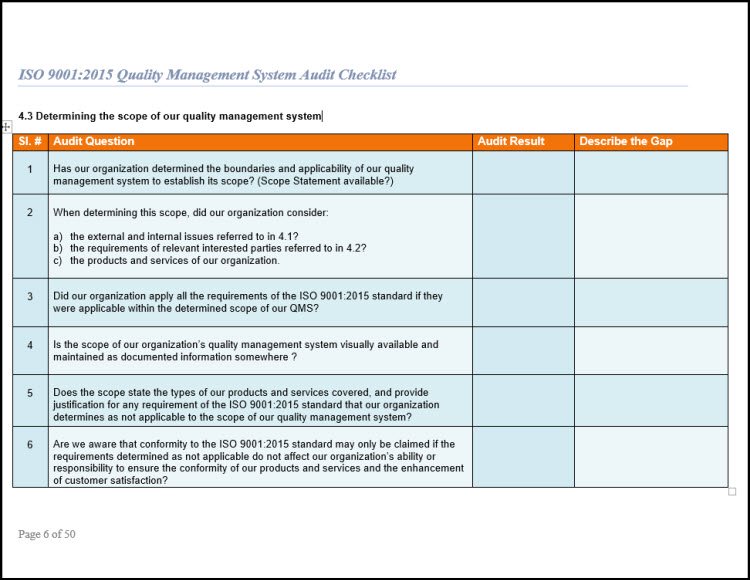
- The scope of the quality management system (clause 4.3).
- Documented information necessary to support the operation of processes (clause 4.4).
- The quality policy (clause 5.).
- The quality objectives (clause 6.2)
- This documented information is subject to the requirements of clause 7.5 for documented information and records control.
- Documented information maintained by the organization for the purpose of communicating the information necessary for the organization to operate (low level, specific documents). See 4.4.
Although ISO 9001:2015 does not specifically requires any of them, examples of documents that can add value to a QMS may include:
- Organization charts
- Process maps, process flow charts and/or process descriptions
- Procedures
- Work and/or test instructions
- Specifications
- Documents containing internal communications
- Production schedules
- Approved supplier lists
- Test and inspection plans
- Quality plans
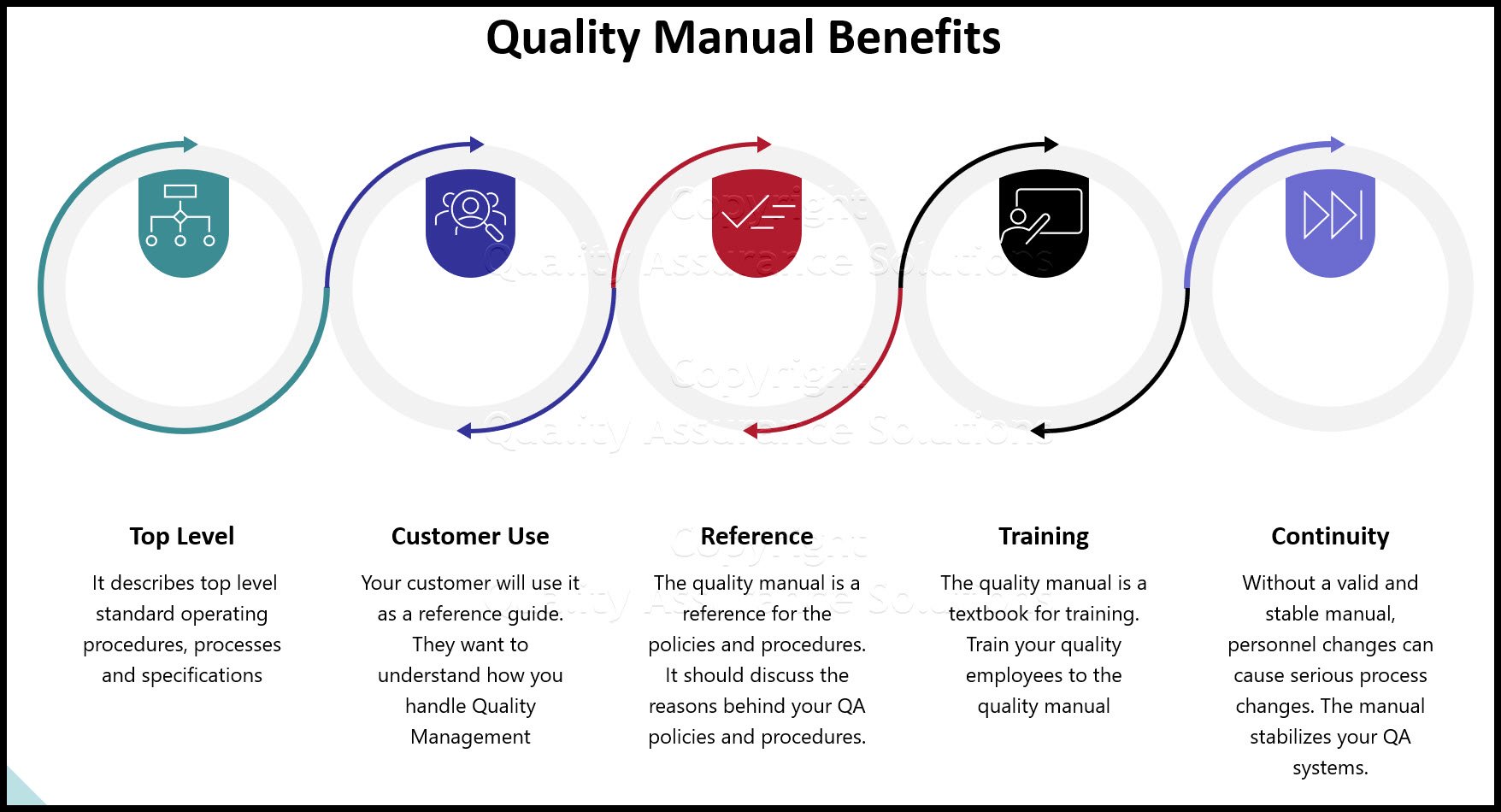
- Quality manuals
- Strategic plans
- Forms
Does this sound simple? Write the above and then you can apply for ISO 9001 certification. Sorry, no it is not that easy. Why not? because there is a catch:
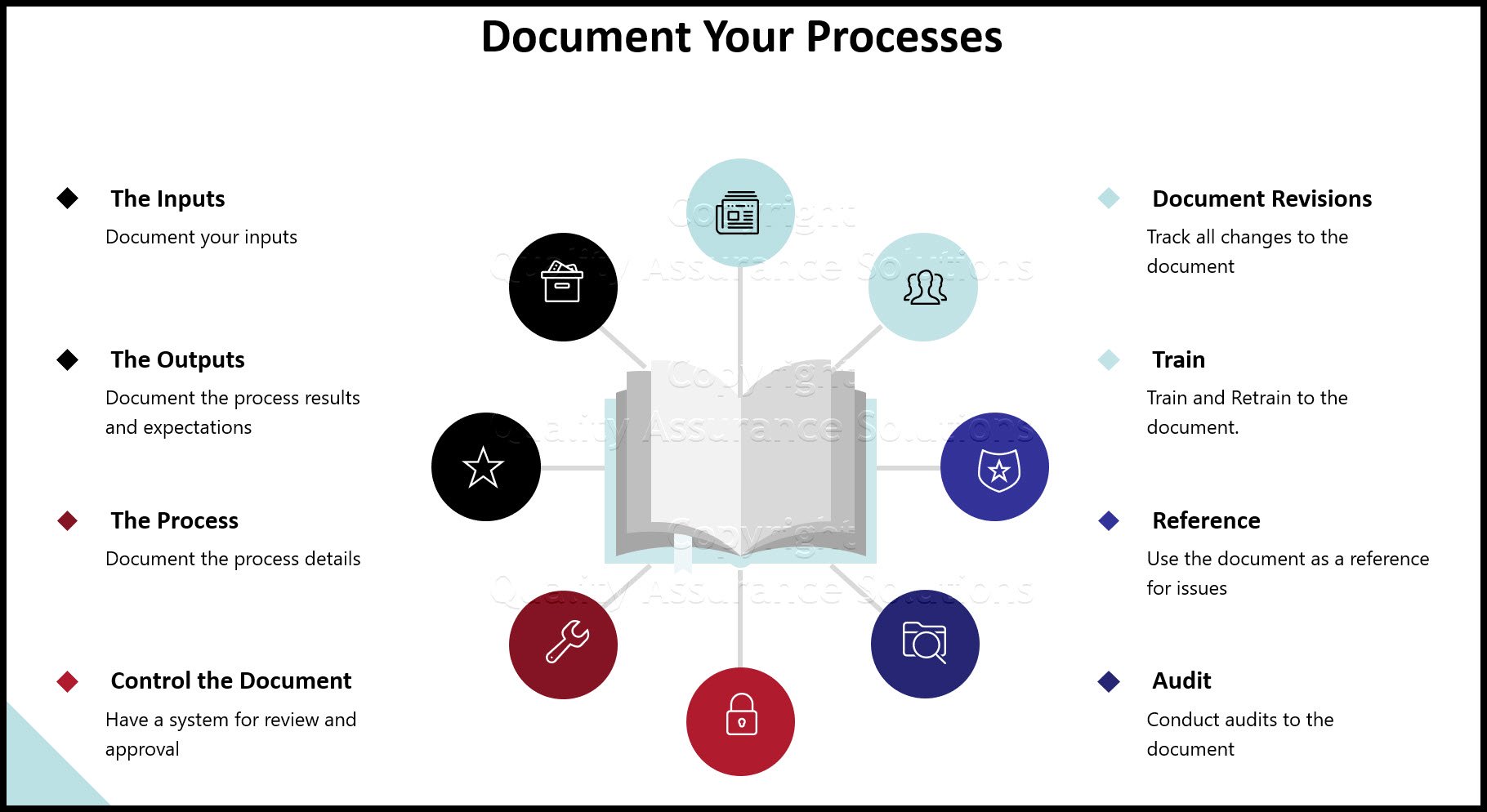
The standard also requires procedures to ensure effective planning, operation and control of all QMS processes.
Your companies processes determine the additional necessary documents. For small companies, you may only need a few more procedures. For large companies with many departments, many products and many processes will require numerous procedures.
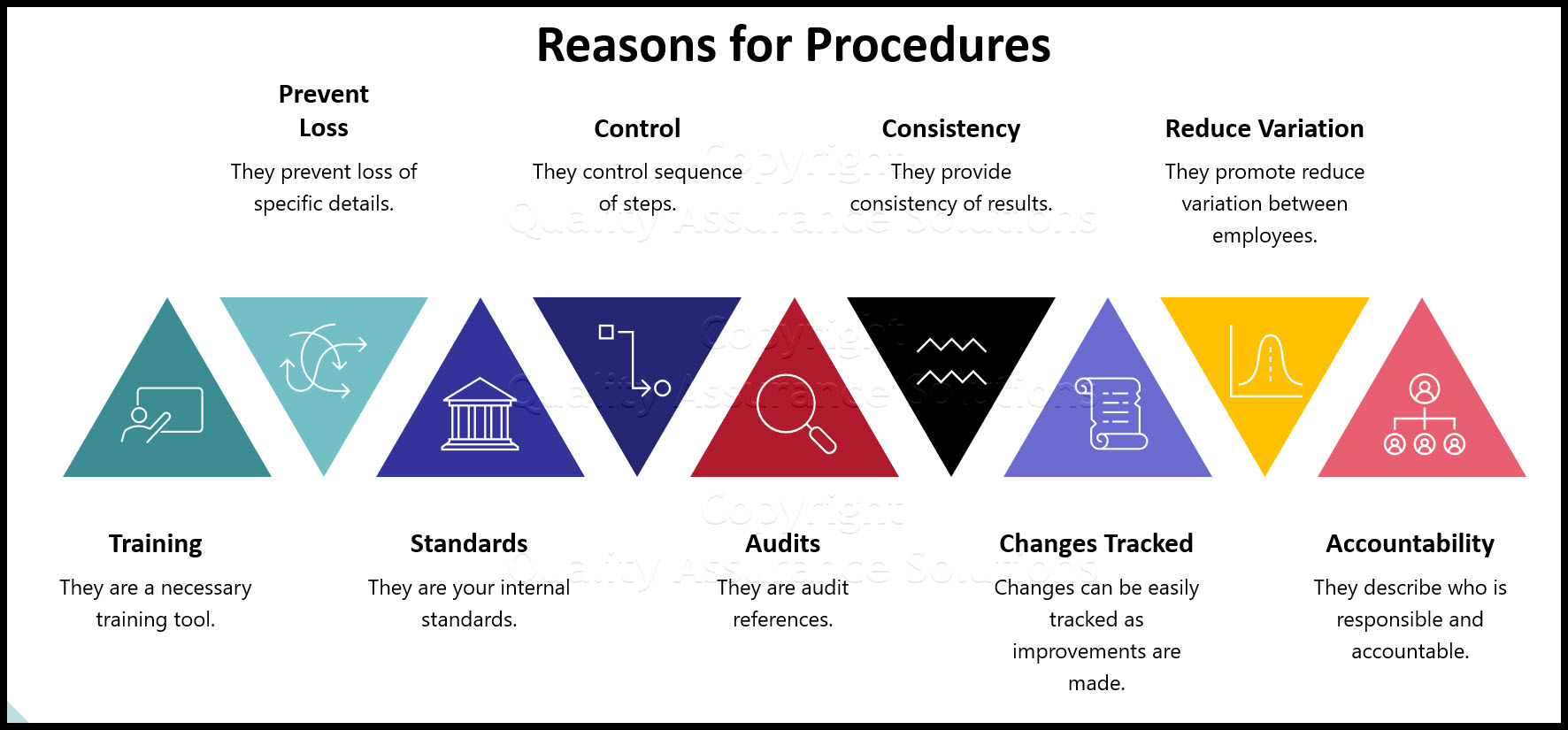
Why do you need procedures? Because
- They prevent loss of specific details.
- They control sequence of steps.
- They provide consistency of results.
- They promote uniformity and reduce variation between employees.
- They are a necessary training tool.
- They are your internal standards.
- They are audit references.
- Changes can be easily tracked as improvements are made.
- They describe who is responsible and accountable.
Cover the 6 Ws in your procedures

- Why must the procedure be followed?
- How is the activity performed?
- What is covered within the procedure?
- When is the procedure applicable?
- Where does the activity take place ?
- Who is responsible for following the procedure?
Make the procedure readable. Make it easy to find the information. Make it logical. Keep is simple.
How do you know which details to include and which details not to include in a procedure? Ask this question about the details. If I leave a specific detail out of the procedure does it affect product quality? If it doesn’t affect product quality then you can leave those details out.
The three tier approach to ISO 9001 2015 procedures
Tier one is the Quality Assurance Manual.
Tier two is the quality assurance management system procedures. This would include the other procedures listed above and any other procedure that specify systems within the Quality Management System.
Tier three is the working documents that detail the specific activity for controlling the product and processes.
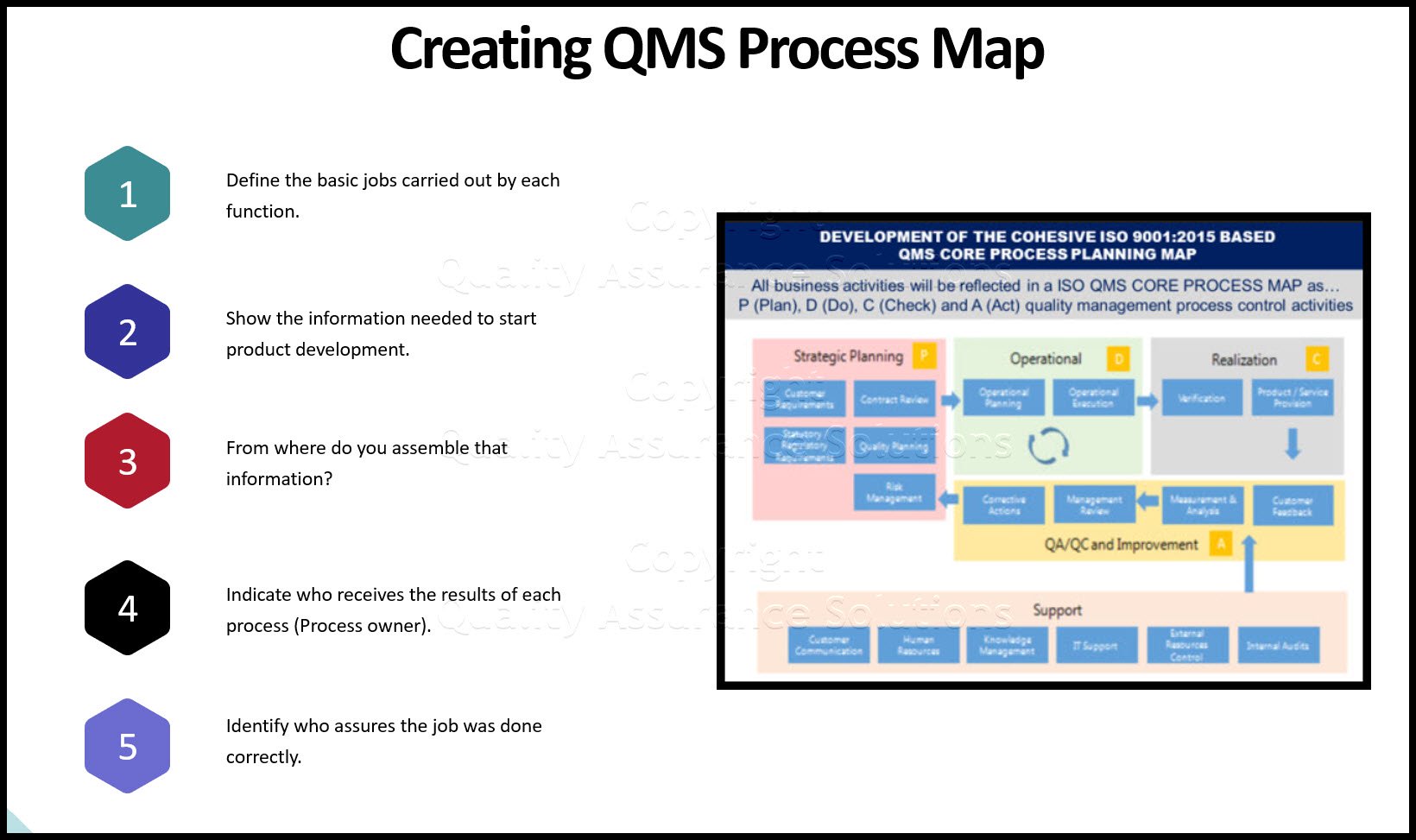
Flowcharting
Many ISO documents can be created by using flowcharts.
|
Quality Assurance Solutions Robert Broughton (805) 419-3344 USA |
 |
|
Software, Videos, Manuals, On-Line Certifications | ||
|
An Organizational Task Management System. Projects, Meetings, Audits & more | ||
|
Corrective Action Software | ||
|
Plan and Track Training | ||
|
AQL Inspection Software |
|
450+ Editable Slides with support links | ||
|
Learn and Train TRIZ | ||
|
Editable Template | ||
|
Templates, Guides, QA Manual, Audit Checklists | ||
|
EMS Manual, Procedures, Forms, Examples, Audits, Videos | ||
|
On-Line Accredited Certifications Six Sigma, Risk Management, SCRUM | ||
|
Software, Videos, Manuals, On-Line Certifications |